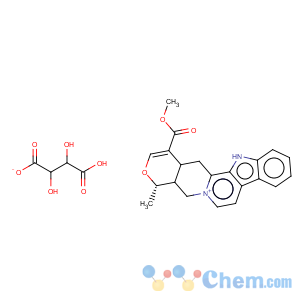
Title: Serpentine (Alkaloid)
CAS Registry Number: 18786-24-8
CAS Name: 3,4,5,6,16,17-Hexadehydro-16-(methoxycarbonyl)-19a-methyloxayohimbanium
Molecular Formula: C21H20N2O3
Molecular Weight: 348.40
Percent Composition: C 72.40%, H 5.79%, N 8.04%, O 13.78%
Literature References: From roots of
Rauwolfia serpentina (L.) Benth.,
Apocynaceae: Schlittler, Schwarz,
Helv. Chim. Acta 33, 1463 (1950). Structure: Schlittler
et al., ibid. 37, 1912 (1954); Klohs
et al., J. Am. Chem. Soc. 76, 1332 (1954); Wenkert, Roychaudhuri,
ibid. 80, 1613 (1958). Stereoisomer of alstonine,
q.v. Stereochemistry: Fritz,
Ann. 655, 148 (1962); Shamma, Richey,
J. Am. Chem. Soc. 85, 2507 (1963).
Properties: Solvated, yellow rods or leaflets from abs ethanol, mp 158° (air-dried), mp 175° (after drying at 120° in a high vacuum, but still not entirely solvent-free). Dec and turns red on drying at 150°. [a]D25 +292° (c = 0.27 in methanol); [a]D25 +267° (c = 0.21 in ethanol). uv max (ethanol): 252, 308, 370 nm (log e 4.49, 4.30, 3.61). Freely sol in methanol. Sol in 10% acetic acid, in other organic solvents.
Melting point: mp 158° (air-dried); mp 175° (after drying at 120° in a high vacuum, but still not entirely solvent-free)
Optical Rotation: [a]D25 +292° (c = 0.27 in methanol); [a]D25 +267° (c = 0.21 in ethanol)
Absorption maximum: uv max (ethanol): 252, 308, 370 nm (log e 4.49, 4.30, 3.61)
Derivative Type: Nitrate
Molecular Formula: C21H20N2O3.HNO3
Molecular Weight: 411.41
Percent Composition: C 61.31%, H 5.14%, N 10.21%, O 23.33%
Properties: Yellow crystals, mp 170-172°. Only slightly sol in water.
Melting point: mp 170-172°
Derivative Type: Hydrochloride monohydrate
Molecular Formula: C21H20N2O3.HCl.H2O
Molecular Weight: 402.87
Percent Composition: C 62.61%, H 5.75%, N 6.95%, O 15.89%, Cl 8.80%
Properties: Yellow crystals, mp 246-248°. [a]D23 +178°. Sol in water.
Melting point: mp 246-248°
Optical Rotation: [a]D23 +178°
Derivative Type: Perchlorate
Molecular Formula: C21H20N2O3.HClO4
Molecular Weight: 448.85
Percent Composition: C 56.19%, H 4.72%, N 6.24%, O 24.95%, Cl 7.90%
Properties: Yellow crystals, mp 255-256°. [a]D22 +185° (c = 0.5 in acetone). uv max (methanol): 252, 306-308, 365-370 nm (log e 4.54, 4.35, 3.68).
Melting point: mp 255-256°
Optical Rotation: [a]D22 +185° (c = 0.5 in acetone)
Absorption maximum: uv max (methanol): 252, 306-308, 365-370 nm (log e 4.54, 4.35, 3.68)