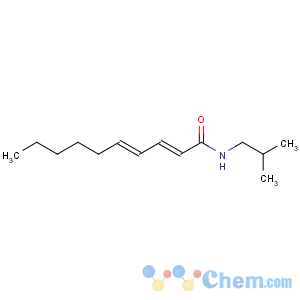
Title: Pellitorine
CAS Registry Number: 18836-52-7
CAS Name: (2
E,4
E)-
N-(2-Methylpropyl)-2,4-decadienamide
Synonyms: (
E,E)
-N-isobutyl-2,4-decadienamide
Molecular Formula: C14H25NO
Molecular Weight: 223.35
Percent Composition: C 75.29%, H 11.28%, N 6.27%, O 7.16%
Literature References: Pungent principle isolated from
Anacyclus pyrethrum DC.,
Compositae: J. M. Gulland, G. U. Hopton,
J. Chem. Soc. 1930, 6. Structure: M. Jacobson,
J. Am. Chem. Soc. 71, 366 (1949). Synthesis:
idem, ibid. 75, 2584 (1953); L. Crombie,
J. Chem. Soc. 1955, 1007; J. Tsuji
et al., Tetrahedron Lett. 1977, 1917; J. Nokami
et al., ibid. 21, 4455 (1980); T. Mandai
et al., Chem. Lett. 1980, 313. Stereoselective synthesis: R. Bloch, D. Hassangonzales,
Tetrahedron 42, 4975 (1986). Insecticidal activity: R. T. Lalonde
et al., J. Chem. Ecol. 6, 35 (1980).
Properties: Needles from petr ether, mp 90°. uv max (abs ethanol): 258 nm (E1%1cm 1330). Sol in organic solvents; sparingly sol in water. Practically insol in dil acid or alkalies.
Melting point: mp 90°
Absorption maximum: uv max (abs ethanol): 258 nm (E1%1cm 1330)