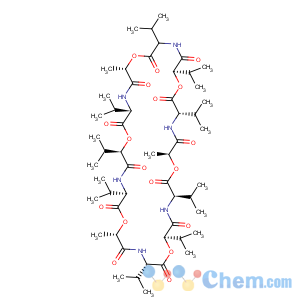
Title: Valinomycin
CAS Registry Number: 2001-95-8
Molecular Formula: C54H90N6O18
Molecular Weight: 1111.32
Percent Composition: C 58.36%, H 8.16%, N 7.56%, O 25.91%
Literature References: Cyclododecadepsipeptide ionophore antibiotic produced by
Streptomyces fulvissimus and related to the enniatins,
q.v. Composed of 3 moles each of L-valine, D-a-hydroxyisovaleric acid, D-valine, and L-lactic acid linked alternately to form a 36-membered ring: Brockmann
et al., Ber. 88, 57 (1955);
Ann. 603, 216 (1957). Structural studies: Shemyakin
et al., Tetrahedron Lett. 1963, 351;
Tetrahedron 19, 995 (1963). Proposed structure: Brockmann
et al., Naturwissenschaften 50, 689 (1963). Structure and synthesis: Shemyakin
et al., Tetrahedron Lett. 1963, 1921. Solid phase synthesis: Gisin
et al., J. Am. Chem. Soc. 91, 2691 (1969); Losse, Klengel,
Tetrahedron 27, 1423 (1971). Biosynthesis: Smirnova
et al., C.A. 73, 97347m (1970); Ristow
et al., FEBS Lett. 42, 127 (1974). Conformation: Ivanov
et al., Biochem. Biophys. Res. Commun. 34, 803 (1969); Onishi, Urry,
ibid. 36, 194 (1969); Duax
et al., Science 176, 911 (1972).
Review: Y. A. Ovchinnokov, V. T. Ivanov, "The Cyclic Peptides: Structure, Conformation, and Function", in
The Proteins vol. V, H. Neurath, R. L. Hill, Eds. (Academic Press, New York, 3rd ed., 1982) pp 563-573.
Properties: Shiny rectangular platelets from dibutyl ether, mp 190° (hot stage). [a]D20 +31.0° (c = 1.6 in benzene). Neutral reaction. Practically insol in water. Freely sol in petr ether, ether, benzene, chloroform, glacial acetic, butyl acetate, acetone. Active
in vitro against
Mycobacterium tuberculosis.
Melting point: mp 190° (hot stage)
Optical Rotation: [a]D20 +31.0° (c = 1.6 in benzene)
Use: Insecticide, nematocide, Patterson, Wright,
US 3520973 (1970 to Am. Cyanamid).