References of Trichothec-9-ene-3,4,8,15-tetrol,12,13-epoxy-, 4,15-diacetate 8-(3-methylbutanoate), (3a,4b,8a)-
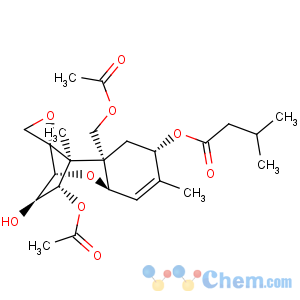
Title: T-2 Toxin
CAS Registry Number: 21259-20-1
CAS Name: (3a,4b,8a)-12,13-Epoxytrichothec-9-ene-3,4,8,15-tetrol 4,15-diacetate 8-(3-methylbutanoate)
Synonyms: 3a-hydroxy-4b,15-diacetoxy-8a-(3-methylbutyryloxy)-12,13-epoxy-D9-tricothecene; 8a-(3-methylbutyryloxy)-4b,15-diacetoxyscirp-9-en-3a-ol; fusariotoxin T-2; insariotoxin; mycotoxin T-2
Manufacturers' Codes: NSC-138780
Molecular Formula: C24H34O9
Molecular Weight: 466.52
Percent Composition: C 61.79%, H 7.35%, O 30.87%
Literature References: Tricothecene mycotoxin isolated from
Fusarium tricinctum: J. R. Bamburg
et al., Tetrahedron 24, 3329 (1968). Physicochemical data: A. E. Pohland
et al., Pure Appl. Chem. 54, 2119 (1982). Synthesis: M. C. Wani
et al., J. Org. Chem. 52, 3468 (1987). Biosynthetic study: F. Van Middlesworth
et al., J. Org. Chem. 55, 1237 (1990). Toxicology studies: W. F. O. Marasas
et al., Toxicol. Appl. Pharmacol. 15, 471 (1969); H. B. Schiefer, D. S. Hancock,
ibid. 76, 464 (1984); D. A. Creasia
et al., Fundam. Appl. Toxicol. 14, 54 (1990). Implicated as a chemical warfare agent in Southeast Asia with nivalenol,
q.v.: N. Wade,
Science 214, 34 (1981); R. T. Rosen, J. D. Rosen,
Biomed. Mass Spectrom. 9, 443 (1982).
Review: Developments in Food Science vol. 4, Y. Ueno, Ed., entitled "Trichothecenes: Chemical, Biological and Toxicological Aspects" (Kodansha Ltd. and Elsevier, New York, 1983) 310 pp. Review of pharmacokinetics and metabolism: B. Yagen, M. Bialer,
Drug Metab. Rev. 25, 281-323 (1993).
Properties: Crystals, mp 151-152°. [a]D26 +15° (c = 2.58 in ethanol). Freely sol in ethyl alcohol, ethyl acetate, chloroform, DMSO and other organic solvents; slightly sol in petroleum ether; very slightly sol in water. LD50 orally in female rats: 4.0 mg/kg (Marasas). LD50 (mg/kg) in mice: 5.2 i.p., 4.2 i.v.; in rats: 7.0 intragastric, 0.9-1.3 i.p., 0.9 i.v., 2.0 s.c.; in guinea pigs: 3.0-4.0 orally, 5.3 intragastric, 1.0 i.m., 1.0-2.0 i.v., 1.0-2.0 s.c.; in pigs: 5.0 orally, 3.0 i.v. (Yagen, Bailer).
Melting point: mp 151-152°
Optical Rotation: [a]D26 +15° (c = 2.58 in ethanol)
Toxicity data: LD50 orally in female rats: 4.0 mg/kg (Marasas); LD50 (mg/kg) in mice: 5.2 i.p., 4.2 i.v.; in rats: 7.0 intragastric, 0.9-1.3 i.p., 0.9 i.v., 2.0 s.c.; in guinea pigs: 3.0-4.0 orally, 5.3 intragastric, 1.0 i.m., 1.0-2.0 i.v., 1.0-2.0 s.c.; in pigs: 5.0 orally, 3.0 i.v. (Yagen, Bailer)
CAUTION: May be highly irritating to skin and mucous membranes. Direct contact may cause extensive inflammation and tissue necrosis (Marasas). Topical exposure has lead to systemic toxicity and death in experimental animals (Schiefer, Hancock).