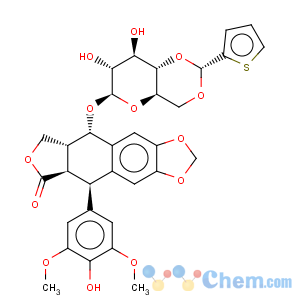
Title: Teniposide
CAS Registry Number: 29767-20-2
CAS Name: (5
R,5a
R,8a
R,9
S)-5,8,8a,9-Tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-[[4,6-
O-[(
R)-2-thienylmethylene]-b-D-glucopyranosyl]oxy]furo[3¢,4¢:6,7]naphtho[2,3-
d]-1,3-dioxol-6(5a
H)-one
Synonyms: 4¢-demethylepipodophyllotoxin 9-(4,6-
O-2-thenylidene-b-D-glucopyranoside); 4¢-demethylepipodophyllotoxin-b-D-thenylidine glucoside; ETP
Manufacturers' Codes: NSC-122819; VM-26
Trademarks: Vehem-Sandoz (Sandoz); Vumon (BMS)
Molecular Formula: C32H32O13S
Molecular Weight: 656.65
Percent Composition: C 58.53%, H 4.91%, O 31.67%, S 4.88%
Literature References: Semi-synthetic derivative of podophyllotoxin,
q.v. Prepn: A. Von Wartburg,
ZA 6607585; C. Keeler-Juslen
et al., US 3524844 (1968, 1970 both to Sandoz). Mechanism of action: H. St?hlen,
Eur. J. Cancer 6, 303 (1970). Pharmacology: M. Hacker, D. Roberts,
Cancer Res. 37, 3287 (1977); S. M. Sieber
et al., Teratology 18, 31 (1978); T. J. Vietti
et al., Cancer Treat. Rep. 62, 1313 (1978). Metabolism: L. Allen,
Drug Metab. Rev. 8, 119 (1978);
Cancer Res. 38, 2549 (1978). Clinical studies: N. M. Gadel-Mawla
et al., Cancer Treat. Rep. 62, 993 (1978); R. E. Bellet
et al., ibid. 445. Studies on delayed toxicity in mice after i.p. injections: M. Hacker, D. Roberts,
Cancer Res. 35, 1756 (1975); H. St?hlin,
Eur. J. Cancer 12, 925 (1976). Review of pharmacology, pharmacokinetics and assay methods: P. I. Clark, M. L. Slevin,
Clin. Pharmacokinet. 12, 223-252 (1987). Comprehensive description: J. J. Kettenes-van den Bosch
et al., Anal. Profiles Drug Subs. 19, 575-600 (1990).
Properties: Crystals from abs ethanol, mp 242-246°. [a]D20 -107° (9:1 chloroform/methanol). uv max (methanol): 283 nm (E1%1cm 64.1). pKa 10.13.
Melting point: mp 242-246°
pKa: pKa 10.13
Optical Rotation: [a]D20 -107° (9:1 chloroform/methanol)
Absorption maximum: uv max (methanol): 283 nm (E1%1cm 64.1)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkaloids/Natural Products; Podophyllum Derivatives; Topoisomerase II Inhibitor.