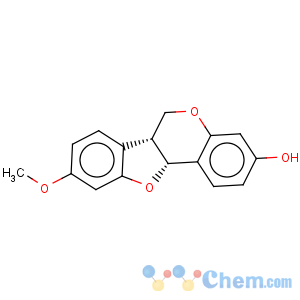
Title: Medicarpin
CAS Registry Number: 32383-76-9
CAS Name: (6a
R,11a
R)-6a,11a-Dihydro-9-methoxy-6
H-benzofuro[3,2-
c][1]benzopyran-3-ol
Synonyms: (-)-3-hydroxy-9-methoxypterocarpan; demethylhomopterocarpin
Molecular Formula: C16H14O4
Molecular Weight: 270.28
Percent Composition: C 71.10%, H 5.22%, O 23.68%
Literature References: Antifungal phytoalexin produced by leguminous species. Isoln from the heartwood of
Swartzia madagascariensis Desv.,
Caesalpinioidae: S. H. Harper
et al., Chem. Ind. (London) 1965, 562; from alfalfa,
Medicago sativa L.,
Leguminosae: D. G. Smith
et al., Physiol. Plant Pathol. 1, 41, (1971); from red clover,
Trifolium pratense L.,
Leguminosae: V. J. Higgins, D. G. Smith,
Phytopathology 62, 235 (1972). 13C-NMR: A. A. Chalmers
et al., Tetrahedron 33, 1735 (1977). HPLC: J. Koster
et al., J. Chromatogr. 270, 392 (1983). Synthesis of racemic form: W. Cocker
et al., J. Chem. Soc. C 1965, 1034. Biosynthetic studies: P. M. Dewick,
Chem. Commun. 1975, 656; S. W. Banks
et al., ibid. 1982, 157; H. A. M. Al-Ani, P. M. Dewick,
J. Chem. Soc. Perkin Trans. 1 1984, 2831. Antifungal properties: L. J. Duczek, V. J. Higgins,
Can. J. Bot. 54, 2620 (1976); H. D. Van Etten,
Phytochemistry 15, 655 (1976); A. O. Latunde-Dada, J. A. Lucas,
Physiol. Plant Pathol. 26, 31 (1985).
Properties: Prisms from benzene, mp 127.5-128.5°. [a]D22 -226° (chloroform). uv max: 207, 282, 287, 310 nm (log e 4.86, 3.97, 4.01, 3.38).
Melting point: mp 127.5-128.5°
Optical Rotation: [a]D22 -226° (chloroform)
Absorption maximum: uv max: 207, 282, 287, 310 nm (log e 4.86, 3.97, 4.01, 3.38)
Derivative Type: (±)-Form
Properties: Prisms from ethyl acetate/light petroleum, mp 194-195°.
Melting point: mp 194-195°