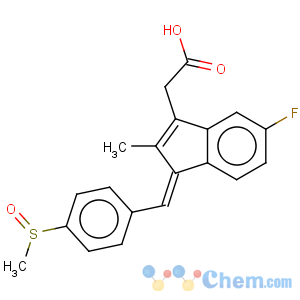
Title: Sulindac
CAS Registry Number: 38194-50-2
CAS Name: (1
Z)-5-Fluoro-2-methyl-1-[[4-(methylsulfinyl)phenyl]methylene]-1
H-indene-3-acetic acid
Synonyms: cis-5-fluoro-2-methyl-1-[
p-(methylsulfinyl)benzylidene]indene-3-acetic acid
Manufacturers' Codes: MK-231
Trademarks: Aflodac (Janus); Algocetil (Francia); Arthrocine (Merck & Co.); Artribid (Merck & Co.); Citireuma (CT); Clinoril (Merck & Co.); Clisundac (Lagap); Reumofil (Ausonia); Reumyl (Lenza); Sudac (Errekappa); Sulinol (ICT); Sulreuma (Von Boch)
Molecular Formula: C20H17FO3S
Molecular Weight: 356.41
Percent Composition: C 67.40%, H 4.81%, F 5.33%, O 13.47%, S 9.00%
Literature References: Non-steroidal anti-inflammatory drug. Prepn: T.-Y. Shen
et al., DE 2039426;
eidem, US 3654349 (1971, 1972 both to Merck & Co.). Stereospecific synthesis: R. F. Shuman
et al., J. Org. Chem. 42, 1914 (1977); enantioselective synthesis: A. R. Maguire
et al., Synlett 2001, 41. 13C-NMR study: A. W. Douglas,
Can. J. Chem. 56, 2129 (1978). Metabolism and disposition: H. B. Hucker
et al., Drug Metab. Dispos. 1, 721 (1973). HPLC determn in biological fluids: D. G. Musson
et al., J. Pharm. Sci. 73, 1270 (1984). Book:
Current Concepts on Anti-inflammatory Drugs, K. Miehlke, Ed. (Biomedical Information Corp., New York, 1980) 240 pp. Review of pharmacology and efficacy in rheumatic disease: R. N. Brogden
et al., Drugs 16, 97-114 (1978); in treatment of colorectal polyps: F. Tonelli
et al., Dig. Dis. 12, 259-264 (1994). Review of clinical pharmacokinetics: N. M. Davies, M. S. Watson,
Clin. Pharmacokinet. 32, 437-459 (1997).
Properties: Yellow odorless crystals from ethyl acetate, mp 182-185° (dec). uv max (methanolic 0.1
N HCl): 327, 285, 256, 226 nm (E1%1cm 375, 420, 410, 540). pKa (25°) 4.7. Sparingly sol in methanol, U.S.P. alcohol; slightly sol in ethyl acetate. Practically insol in water at pH <4.5. Soly increases with rising pH to ~3.0 mg/ml at pH 7. Stable in aq acid and base. Solid stable for at least three days in air at 100°.
Melting point: mp 182-185° (dec)
pKa: pKa (25°) 4.7
Absorption maximum: uv max (methanolic 0.1
N HCl): 327, 285, 256, 226 nm (E1%1cm 375, 420, 410, 540)
Therap-Cat: Anti-inflammatory.
Keywords: Anti-inflammatory (Nonsteroidal); Arylacetic Acid Derivatives.