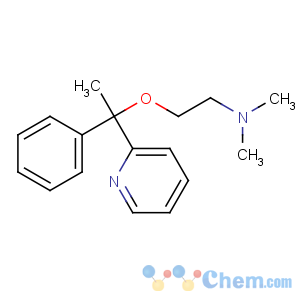
Title: Doxylamine
CAS Registry Number: 469-21-6
CAS Name: N,N-Dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine
Synonyms: 2-[a-(2-dimethylaminoethoxy)-a-methylbenzyl]pyridine; phenyl-2-pyridylmethyl-b-
N,N-dimethylaminoethyl ether; 2-dimethylaminoethoxyphenylmethyl-2-picoline
Molecular Formula: C17H22N2O
Molecular Weight: 270.37
Percent Composition: C 75.52%, H 8.20%, N 10.36%, O 5.92%
Literature References: Prepn: C. H. Tilford
et al., J. Am. Chem. Soc. 70, 4001 (1948); N. Sperber
et al., ibid. 71, 887 (1949). Pharmacology: B. B. Brown, H. Werner,
J. Lab. Clin. Med. 33, 325 (1948). GC determn: H. C. Thompson
et al., J. Chromatogr. Sci. 20, 373 (1982). Chronic toxicity study: C. D. Jackson, B. Blackwell,
J. Am. Coll. Toxicol. 12, 1 (1993). Review of properties and pharmacology: T. J. Haley,
Dangerous Prop. Ind. Mater. Rep. 2, 17-20 (1982). Clinical evaluation as hypnotic: F. Sj?qvist, L. Lasagna,
Clin. Pharmacol. Ther. 8, 48 (1967); as antihistaminic for use in colds: R. Eccles
et al., J. Pharm. Pharmacol. 47, 990 (1995).
Properties: Liquid, bp0.5 137-141°. Sol in acids. Slightly volatile, darkens on exposure to light.
Boiling point: bp0.5 137-141°
Derivative Type: Succinate
CAS Registry Number: 562-10-7
Trademarks: Gittalun (Boehringer, Ing.); Hoggar (Stada); Mereprine (Cassella-med); Sedaplus (Rosen Pharma); Unisom (Pfizer)
Molecular Formula: C17H22N2O.C4H6O4
Molecular Weight: 388.46
Percent Composition: C 64.93%, H 7.27%, N 7.21%, O 20.59%
Properties: Crystals, mp 100-104°, sol in water. One gram dissolves in 1 ml water, 2 ml alcohol, 2 ml chloroform. Slightly sol in benzene and ether. pH (1% aq soln): 4.9 to 5.1. LD50 in mice, rabbits (mg/kg): 470, 250 orally; 62, 49 i.v.; in mice, male rats, female rats (mg/kg): 460, 440, 445 s.c. (Brown, Werner).
Melting point: mp 100-104°
Toxicity data: LD50 in mice, rabbits (mg/kg): 470, 250 orally; 62, 49 i.v.; in mice, male rats, female rats (mg/kg): 460, 440, 445 s.c. (Brown, Werner)
Derivative Type: Combination with pyridoxine hydrochloride
Trademarks: Bendectin (Duchesnay); Diclectin (Duchesnay)
Literature References: Has been used for nausea of pregnancy. Some formulations also contained dicyclomine,
q.v. Review of therapeutic use and the issue of teratogenicity: L. J. Sheffield, R. Batagol,
Med. J. Aust. 143, 143-147 (1985); R. L. Brent,
Reprod. Toxicol. 9, 337-349 (1995).
Therap-Cat: Antihistaminic; sedative, hypnotic.
Therap-Cat-Vet: Antihistaminic.
Keywords: Antihistaminic; Aminoalkylethers; Sedative/Hypnotic.