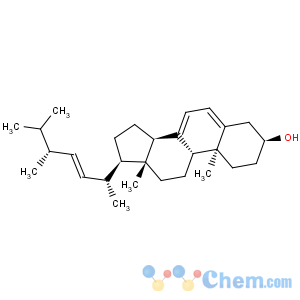
Title: Lumisterol
CAS Registry Number: 474-69-1
CAS Name: (3b,9b,10a,22
E)-Ergosta-5,7,22-trien-3-ol
Molecular Formula: C28H44O
Molecular Weight: 396.65
Percent Composition: C 84.78%, H 11.18%, O 4.03%
Literature References: Differs from ergosterol by spatial arrangement involving the methyl group at C-10. Prepd by ultraviolet irradiation of a benzene-alcohol soln of ergosterol: Askew
et al., Proc. Roy. Soc. (London) B109, 488 (1932). Stereochemistry: Castells
et al., J. Chem. Soc. 1959, 1159. Dehydrogenation with selenium gives Diel's hydrocarbon, 3¢-methyl-1,2-cyclopentenophenanthrene.
Properties: Needles from acetone-methanol, mp 118°. [a]D19 +191.5°, [a]19546 +235.4° (c = 2 in acetone). uv max: 265, 280 nm: Windaus
et al., Ann. 493, 259 (1932); Heilbron
et al., J. Chem. Soc. 1937, 411. Practically insol in water. Sol in organic solvents. Forms a monomolecular compd with calciferol, mp 122°.
Melting point: mp 118°; mp 122°
Optical Rotation: [a]D19 +191.5°; [a]19546 +235.4° (c = 2 in acetone)
Absorption maximum: uv max: 265, 280 nm: Windaus
et al., Ann. 493, 259 (1932); Heilbron
et al., J. Chem. Soc. 1937, 411
Derivative Type: Acetate
Molecular Formula: C30H46O2
Molecular Weight: 438.69
Percent Composition: C 82.14%, H 10.57%, O 7.29%
Properties: mp 100°. [a]D19 +130.5°, [a]19546 +163° (c = 1.8 in acetone).
Melting point: mp 100°
Optical Rotation: [a]D19 +130.5°; [a]19546 +163° (c = 1.8 in acetone)