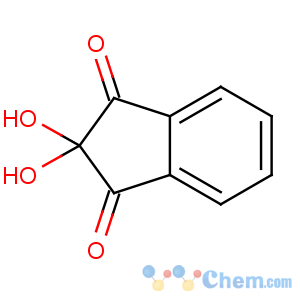
Title: Ninhydrin
CAS Registry Number: 485-47-2
CAS Name: 2,2-Dihydroxy-1
H-indene-1,3(2
H)-dione
Synonyms: 2,2-dihydroxy-1,3-indanedione; 1,2,3-indantrione monohydrate; triketohydrinden hydrate
Molecular Formula: C9H6O4
Molecular Weight: 178.14
Percent Composition: C 60.68%, H 3.39%, O 35.93%
Literature References: Prepn: Ruhemann,
J. Chem. Soc. 97, 1438 (1910); Teeters, Shriner,
J. Am. Chem. Soc. 55, 3026 (1933); Wanag, Lode,
Ber. 71, 1267 (1938). Improved syntheses: L. F. Fieser,
Experiments in Organic Chemistry (Boston, 3rd ed., 1955) p 123; Becker, Russell,
J. Org. Chem. 28, 1896 (1963). Absorption spectrum: Polonovski
et al., Bull. Soc. Chim. [5]
6, 1557 (1939). Poisonous action on bacteria, mice, guinea pigs: O. Loew,
Biochem. Z. 69, 111 (1915). Acute toxicity study: J. C. Breton
et al., C.R. Seances Soc. Biol. Ses Fil. 151, 719 (1957). Monograph: Henri Plagnol,
Influence de la Structure des Composés Aminés dans leur Reaction avec la Ninhydrine (Bordeaux, 1962) 156 pp.
Reviews: J. C. Breton,
Etudes Chimiques et Biologiques sur la Ninhydrine, Réactif des Aminoacides (Imprimerie E. Drouillard, Bordeaux, 1958) 285 pp; McCaldin,
Chem. Rev. 60, 39 (1960); A. Schoenberg, E. Singer,
Tetrahedron 34, 1285-1300 (1978).
Derivative Type: Monohydrate
Properties: Pale yellow prisms from water or alcohol. Reddens at 125°, swells at 139°, dec 241°. Freely sol in water. LD50 i.p. in mice: 78 mg/kg (Breton, 1957).
Toxicity data: LD50 i.p. in mice: 78 mg/kg (Breton, 1957)
Use: Reagent for the detection of free amino and carboxyl groups in proteins and peptides, yielding a blue color under the proper conditions.