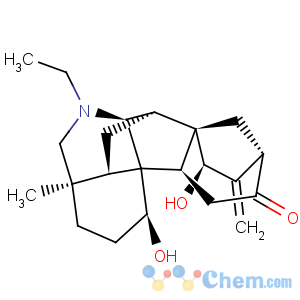
Title: Songorine
CAS Registry Number: 509-24-0
CAS Name: (1a,15b)-21-Ethyl-1,15-dihydroxy-4-methyl-16-methylene-7,20-cycloveatchan-12-one
Synonyms: napellonine; zongorine
Molecular Formula: C22H31NO3
Molecular Weight: 357.49
Percent Composition: C 73.91%, H 8.74%, N 3.92%, O 13.43%
Literature References: From
Aconitum songoricum Popov,
Ranunculaceae: Yunusov,
J. Gen. Chem. USSR 18, 515 (1948); Kuzovkov,
ibid. 23, 504 (1953);
25, 2006 (1955). Identity with napellonine: Kuzovkov,
Zh. Obshch. Khim. 28, 2283 (1958);
29, 1728 (1959). Structure: Sugasawa,
Chem. Pharm. Bull. 9, 889, 897 (1961). Absolute configuration: Okamoto
et al., ibid. 13, 1270 (1965). Synthesis of the aromatic intermediate: Wiesner
et al., Can. J. Chem. 51, 3978 (1973). Pharmacology: Sadritdinov,
Farmakol. Alk. No. 312 (1965),
C.A. 66, 93772d (1967). Mass spectra data: Yunusov
et al., Khim. Prir. Soedin. 6, 101 (1970),
C.A. 73, 131178u (1970). Pharmacology and toxicity data: N. G. Bisset,
J. Ethnopharmacol. 4, 247-336 (1981).
Properties: Crystals, mp 201-202°. [a]D20 -135.4°. uv max: 290 nm (log e 2.6). LD50 in mice (mg/kg): 1575 orally, 630 s.c., 485 i.p., 142.5 i.v.; in rats (mg/kg): 407.5 i.p. (Bisset).
Melting point: mp 201-202°
Optical Rotation: [a]D20 -135.4°
Absorption maximum: uv max: 290 nm (log e 2.6)
Toxicity data: LD50 in mice (mg/kg): 1575 orally, 630 s.c., 485 i.p., 142.5 i.v.; in rats (mg/kg): 407.5 i.p. (Bisset)
Derivative Type: Hydrochloride
Properties: Crystals, mp 257-258°. [a]D20 -114° (c = 2 in water).
Melting point: mp 257-258°
Optical Rotation: [a]D20 -114° (c = 2 in water)