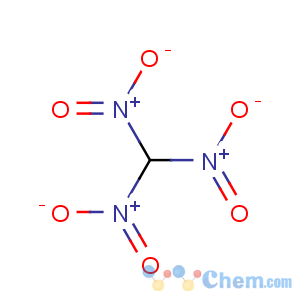
Title: Trinitromethane
CAS Registry Number: 517-25-9
Synonyms: Nitroform
Molecular Formula: CHN3O6
Molecular Weight: 151.04
Percent Composition: C 7.95%, H 0.67%, N 27.82%, O 63.56%
Line Formula: CH(NO2)3
Literature References: Prepn from tetranitromethane and K4[Fe(CN)6] in aq soln: Chattaway, Harrison,
J. Chem. Soc. 109, 171 (1916); by nitration of acetylene with nitric acid: Hager,
Ind. Eng. Chem. 41, 2168 (1949).
Properties: Crystals, mp 15° (the unstable
aci-form, mp 50°). d425 (liq) 1.469. Heat of combustion 746 cal/g. Dipole moment 2.61 (benzene). Dec above 25°. Explodes when heated rapidly. Sol in water, giving an intensely yellow soln, although the dry crystals are pure white.
Melting point: mp 15°; mp 50°
Density: d425 (liq) 1.469
Derivative Type: Potassium salt
Molecular Formula: CK(NO2)3
Molecular Weight: 189.13
Percent Composition: C 6.35%, K 20.67%, N 22.22%, O 50.76%
Properties: Moderately stable crystals. Soly in water at 0°: 16.7 g/100 ml; at 60°: 193.8 g/100 ml. Soly in ethanol: 5.29 g/l.
CAUTION: Slightly irritating to eyes, mucous membranes.
Use: In the manuf of explosives and propellants.