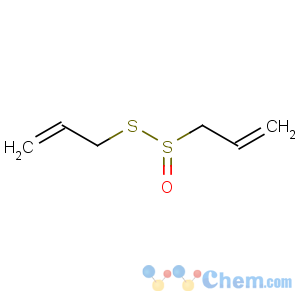
Title: Allicin
CAS Registry Number: 539-86-6
CAS Name: 2-Propene-1-sulfinothioic acid
S-2-propenyl ester
Synonyms: thio-2-propene-1-sulfinic acid
S-allyl ester; diallyl disulfide-oxide
Molecular Formula: C6H10OS2
Molecular Weight: 162.27
Percent Composition: C 44.41%, H 6.21%, O 9.86%, S 39.52%
Literature References: A biologically active constit of freshly crushed garlic
(Allium sativum L.,
Liliaceae). Naturally formed by the action of the enzyme allicinase on alliin,
q.v., when the tissue of the garlic bulb is disrupted. Isoln and antibacterial activity: C. J. Cavallito, J. H. Bailey,
J. Am. Chem. Soc. 66, 1950 (1944). Structure: Cavallito
et al., ibid. 1952. Synthesis: Stoll, Seebeck,
Experientia 6, 330 (1950); Cavallito, Small,
US 2508745 (1950 to Sterling Drug). Antifungal activity: Y. Yamada, K. Azuma,
Antimicrob. Agents Chemother. 11, 743 (1977). Pharmacological effects and prepn: P. R. Mayeux
et al., Agents Actions 25, 182 (1988). Stability in blood, solvents, and simulated physiological fluids: F. Freeman, Y. Kodera,
J. Agric. Food Chem. 43, 2332 (1995). Identification as the source of garlic's pungent burning and prickling sensations: L. J. Macpherson
et al., Curr. Biol. 15, 929 (2005).
Properties: Yellow liquid. True odor of garlic. Decomp on distilling. d420 1.112.
nD20 1.561. Soly in water at 10° about 2.5% w/w. pH about 6.5. Upon standing an oily precipitate forms from aq solns. Miscible with alcohol, ether, benzene; fairly insol in the Skellysolves; unstable to hot alkali; stable to acids. LD50 in mice (mg/kg): 60 i.v.; 120 s.c. (Cavallito, Bailey).
Index of refraction: nD20 1.561
Density: d420 1.112
Toxicity data: LD50 in mice (mg/kg): 60 i.v.; 120 s.c. (Cavallito, Bailey)