Title: Cellocidin
CAS Registry Number: 543-21-5
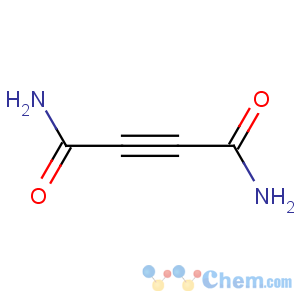
CAS Name: 2-Butynediamide
Synonyms: acetylenedicarboxamide; acetylenedicarboxylic acid diamide; aquamycin; lenamycin
Molecular Formula: C4H4N2O2
Molecular Weight: 112.09
Percent Composition: C 42.86%, H 3.60%, N 24.99%, O 28.55%
Line Formula: H2NCOCoCCONH2
Literature References: Antibiotic substance with antibacterial activity. Produced by
Streptomyces chibaensis from soil collected at Chiba City, Japan: Suzuki
et al., J. Antibiot. 11, 81 (1958). Synthesis from dimethyl acetylenedicarboxylate and concd ammonium hydroxide at -10°: Saggiomo,
J. Org. Chem. 22, 1171 (1957); Suzuki, Okuma,
J. Antibiot. 11, 84 (1958). Identity with lenamycin: Y. Sekizawa,
Meiji Seika Kenkyu Nempo 1960, 42,
C.A. 56, 14609a (1962). Biosynthesis: E. R. H. Jones,
J. Chem. Soc. Perkin Trans. 1 1973, 148.
Properties: Crystals from dil methanol, dec. 216-218°. uv max (0.1
N NaOH): 299 nm (E1%1cm 290). Sparingly sol in water, methanol, ethanol, acetone, chloroform, glacial acetic acid. Relatively stable in neutral or acid solns, showing no loss of activity at pH 2 to 7 when heated for 10 min at 100°. Unstable in alkaline soln evolving ammonia. LD50 i.v. in mice: 11 mg/kg (Suzuki).
Absorption maximum: uv max (0.1
N NaOH): 299 nm (E1%1cm 290)
Toxicity data: LD50 i.v. in mice: 11 mg/kg (Suzuki)