Title: Busulfan
CAS Registry Number: 55-98-1
CAS Name: 1,4-Butanediol dimethanesulfonate esters
Synonyms: 1,4-bis(methanesulfonoxy)butane; 1,4-di(methanesulfonyloxy)butane; 1,4-di(methylsulfonoxy)butane; methanesulfonic acid tetramethylene ester; tetramethylene bis(methanesulfonate); busulphan
Manufacturers' Codes: CB-2041; GT-41
Trademarks: Busulfex (Orphan Med.); Misulban (Nuovo ISM); Myleran (GSK)
Molecular Formula: C6H14O6S2
Molecular Weight: 246.30
Percent Composition: C 29.26%, H 5.73%, O 38.98%, S 26.04%
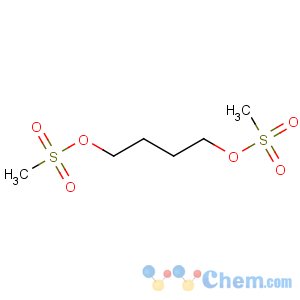
Line Formula: CH3SO2O(CH2)4OSO2CH3
Literature References: Alkylating agent with antileukemic activity. Discovery: A. Haddow, G. M. Timmis,
Lancet 1, 207 (1953). Prepn: G. M. Timmis,
US 2917432 (1959 to Burroughs Wellcome). Chemosterilant effect in boll weevils: J. W. Haynes
et al., J. Econ. Entomol. 66, 619 (1973); J. W. Haynes, J. E. Wright,
Southwest. Entomol. 7, 56 (1982). Pharmacokinetics: H. Ehrsson
et al., Clin. Pharmacol. Ther. 34, 86 (1983). HPLC determn in human plasma: W. D. Henner
et al., J. Chromatogr. 416, 426 (1987). Clinical pretreatment with cyclophosphamide,
q.v., for bone marrow transplants: G. W. Santos
et al., N. Engl. J. Med. 309, 1347 (1983). Comparative clinical trial with mitobronitol,
q.v., in chronic myeloid leukemia: R. T. Silver
et al., Cancer 60, 1442 (1987). Toxicity data: H. R. Scherf
et al., Arzneim.-Forsch. 20, 1467 (1970). Review of pharmacology: C. D. R. Dunn,
Exp. Hematol. 2, 101-117 (1974); of toxicology: J. B. Bishop, J. S. Wassom,
Mutat. Res. 168, 15-45 (1986). Comprehensive description: M. Tariq, A. A. Al Badr,
Anal. Profiles Drug Subs. 16, 53-83 (1987).
Properties: Crystals, mp 114-118°. Soly in acetone at 25°: 2.4 g/100 ml; in alcohol: 0.1 g/100 ml. Practically insol in water, but will dissolve slowly as hydrolysis takes place. LD50 i.v. in rats: 1.8 mg/kg (Scherf).
Melting point: mp 114-118°
Toxicity data: LD50 i.v. in rats: 1.8 mg/kg (Scherf)
CAUTION: This substance is listed as a known human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-39.
Use: Insect sterilant.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkylating Agents; Alkyl Sulfonates.