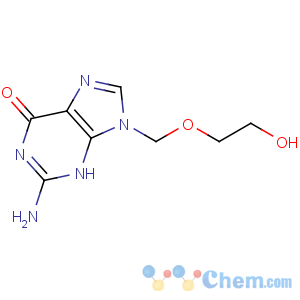
Title: Acyclovir
CAS Registry Number: 59277-89-3
CAS Name: 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6
H-purin-6-one
Synonyms: acycloguanosine; 9-[(2-hydroxyethoxy)methyl]guanine
Manufacturers' Codes: BW-248U; Wellcome 248U
Trademarks: Acicloftal (Bruschettini); Avirase (Lampugnani); Cycloviran (Sigma-Tau); Maynar (Ferrer); Virmen (Menarini); Viruseen (Hommel); Zoliparin (Mann); Zovir (GSK); Zovirax (GSK)
Molecular Formula: C8H11N5O3
Molecular Weight: 225.20
Percent Composition: C 42.67%, H 4.92%, N 31.10%, O 21.31%
Literature References: Orally active acyclic nucleoside with inhibitory activity towards several herpes viruses. Prepn: H. J. Schaeffer,
DE 2539963;
idem, US 4199574 (1976, 1980 to Wellcome). Convenient synthesis from guanine: H. Matsumoto
et al., Chem. Pharm. Bull. 36, 1153 (1988). Selectivity of action: G. B. Elion
et al., Proc. Natl. Acad. Sci. USA 74, 5716 (1977). Chemistry, antiviral activity, metabolism: H. J. Schaeffer
et al., Nature 272, 583 (1978).
In vitro activity: P. Collins, D. J. Bauer,
J. Antimicrob. Chemother. 5, 431 (1979). Effect on herpes simplex infections in mice: H. J. Field
et al., Antimicrob. Agents Chemother. 15, 554 (1979); on herpes zoster in immunocompromised patients: H. H. Balfour
et al., N. Engl. J. Med. 308, 1448 (1983). Treatment of primary episodes of genital herpes simplex infection: Y. J. Bryson
et al., ibid. 916; of recurrent genital herpes: S. E. Straus
et al., ibid. 310, 1545 (1984); J. M. Douglas
et al., ibid. 1551. HPLC determn in serum and clinical pharmacokinetics: G. Bahrami
et al.,
J. Chromatogr. B 816, 327 (2005). Symposia on pharmacology and clinical studies:
Am. J. Med. 73, Suppl. 1A, 1-392 (1982);
J. Antimicrob. Chemother. 12, Suppl. B, 1-202 (1983);
Scand. J. Infect. Dis. Suppl. 47, 1-176 (1985).
Review: R. J. Whitley, J. W. Gnann, Jr.,
N. Engl. J. Med. 327, 782-789 (1992).
Properties: Crystals from methanol, mp 256.5-257°. LD50 in mice (mg/kg): >10,000 orally; 1000 i.p. (Schaeffer).
Melting point: mp 256.5-257°
Toxicity data: LD50 in mice (mg/kg): >10,000 orally; 1000 i.p. (Schaeffer)
Therap-Cat: Antiviral.
Keywords: Antiviral; Purines/Pyrimidinones.