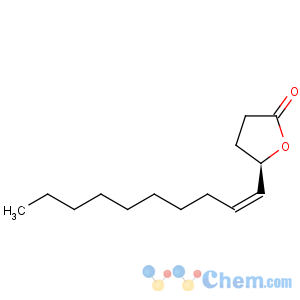
Title: Japonilure
CAS Registry Number: 64726-91-6
CAS Name: [
R-
(Z)]-5-(1-Decenyl)dihydro-2(3
H)-furanone
Synonyms: [(4
R,5
Z)]-tetradecen-4-olide; (
R,Z)-5-(dec-1-enyl)oxacyclopentan-2-one; IN-60
Molecular Formula: C14H24O2
Molecular Weight: 224.34
Percent Composition: C 74.95%, H 10.78%, O 14.26%
Literature References: Sex pheromone produced by the female Japanese beetle,
Popillia japonica Newman (Coleoptera: Scarabaeidae); biological activity is inhibited by its antipode. Isoln and synthesis: J. H. Tumlinson
et al., Science 197, 789 (1977). Description of attractant activity: J. H. Tumlinson in
Adv. Pest. Sci., 4th Int. Congr. Pest. Chem. 2, H. Geissbühler, Ed (Pergamon Press, Oxford, England, 1979) 315-322. Use in beetle traps: T. L. Ladd, Jr., M. G. Klein,
J. Econ. Entomol. 79, 84 (1986); A. Martins
et al., Ecol. Bull. 39, 101 (1988). Stereospecific synthesis: S. Chattopadhyay
et al., Synth. Commun. 20, 1299 (1990); T. Ebata
et al., Biosci. Biotech. Biochem. 56, 818 (1992). Isolation from
Anomala spp. as component of pheromone system and field evaluation: W. S. Leal
et al., J. Chem. Ecol. 20, 1643 (1994); W. S. Leal
et al., ibid. 1667.
Properties: bp0.1mm 130-134°. [a]D21 -69.0° (c = 1.42 in CHCl3). [a]D25 -70.2° (c = 0.5 in CHCl3).
Boiling point: bp0.1mm 130-134°
Optical Rotation: [a]D21 -69.0° (c = 1.42 in CHCl3); [a]D25 -70.2° (c = 0.5 in CHCl3)