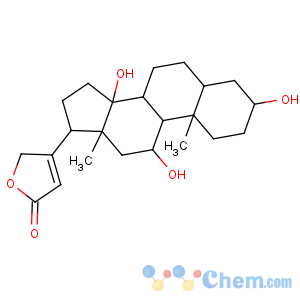
Title: Sarmentogenin
CAS Registry Number: 76-28-8
CAS Name: (3b,5b,11a)-3,11,14-Trihydroxy-card-20(22)-enolide
Molecular Formula: C23H34O5
Molecular Weight: 390.51
Percent Composition: C 70.74%, H 8.78%, O 20.49%
Literature References: An aglucon from sarmentocymarin isolated from the seeds of
Strophanthus sarmentosus DC. var.
senegambiae (A. DC.) Monachino,
Apocynaceae: Jacobs, Heidelberger,
J. Biol. Chem. 81, 765 (1929); Katz,
Helv. Chim. Acta 31, 993 (1948). Structure: Callow, Taylor,
J. Chem. Soc. 1952, 2299; Repke, Klesczewski,
Arch. Exp. Pathol. Pharmakol. 239, 131 (1960).
Properties: Prisms from methanol or methanol + acetone, mp 278-282°. [a]D19 +21.1 ± 4° (c = 0.521 in methanol). Absorption max (98% H2SO4): 230, 415 nm (E1%1cm 460, 430). Sol in alcohol, methanol, pyridine; sparingly sol in acetone, chloroform; practically insol in benzene, ether. Dissolves in concd H2SO4 with a bright golden color which slowly turns green and then indigo. In dil pyridine soln gives a deep red color with alkaline nitroprusside soln. When recrystallized from pyridine, it forms long microplatelets contg ~1 mol of the solvent and melting at 258° with effervescence. Is not precipitated with digitonin. Yields sarmentogenone on oxidation.
Melting point: mp 278-282°; melting at 258°
Optical Rotation: [a]D19 +21.1 ± 4° (c = 0.521 in methanol)
Absorption maximum: Absorption max (98% H2SO4): 230, 415 nm (E1%1cm 460, 430)
Derivative Type: Diacetate
Molecular Formula: C27H38O7
Molecular Weight: 474.59
Percent Composition: C 68.33%, H 8.07%, O 23.60%
Properties: Needles from abs ether, mp 135-155°. Not affected by CrO3 in acetic acid.
Melting point: mp 135-155°
Derivative Type: Dibenzoate
Molecular Formula: C37H42O7
Molecular Weight: 598.73
Percent Composition: C 74.22%, H 7.07%, O 18.71%
Properties: Flat hexagonal prisms from acetone, mp 281°. [a]D20 +14° (acetone). Sparingly sol in alc; practically insol in ether. Not affected by CrO3 in acetic acid.
Melting point: mp 281°
Optical Rotation: [a]D20 +14° (acetone)
Status: This monograph has been retired and is no longer subject to revision or update.