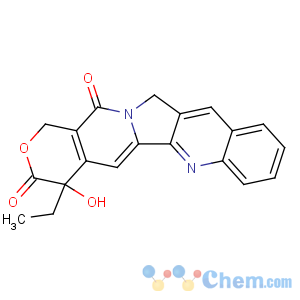
Title: Camptothecin
CAS Registry Number: 7689-03-4
CAS Name: (S)-4-Ethyl-4-hydroxy-1
H-pyrano[3¢,4¢:6,7]indolizino[1,2-
b]quinoline-3,14(4
H,12
H)-dione
Molecular Formula: C20H16N2O4
Molecular Weight: 348.35
Percent Composition: C 68.96%, H 4.63%, N 8.04%, O 18.37%
Literature References: Antitumor alkaloid; prototype DNA topoisomerase I inhibitor. Isoln from the stem wood of the Chinese tree,
Camptotheca acuminata Decsne.,
Nyssaceae, and structure: M. E. Wall
et al., J. Am. Chem. Soc. 88, 3888 (1966). Approach to synthesis: Kepler
et al., J. Org. Chem. 34, 3853 (1969). Total synthesis: E. J. Corey
et al., ibid. 40, 2140 (1975). Total synthesis of racemate: Stork, Schultz,
J. Am. Chem. Soc. 93, 4074 (1971); Volkmann
et al., ibid. 5576; Tang
et al., ibid. 97, 159 (1975); J. C. Bradley, G. Buchi,
J. Org. Chem. 41, 699 (1976); T. Kametani
et al., J. Chem. Soc. Perkin Trans. 1 1981, 1563. Pharmacologic and clinical evaluation: Gottlieb
et al., Cancer Chemother. Rep. Part 1 54, 461 (1970); Gallo
et al., J. Natl. Cancer Inst. 46, 789 (1971); S. M. Sieber
et al., Cancer Treat. Rep. 60, 1127 (1976). Mechanism of action: Y. H. Hsiang
et al., J. Biol. Chem. 260, 14873 (1985). HPLC determn in plasma: J. H. Beijnen
et al., J. Chromatogr. 617, 111 (1993).
Reviews: M. Potmesil,
Cancer Res. 54, 1431-1439 (1994); M. E. Wall, M. C. Wani,
ibid. 55, 753-760 (1995).
Properties: Pale yellow needles from methanol + acetonitrile, dec 264-267°. Also reported as mp 275-277° (Volkmann); 287-288° (Stork, Schultz). [a]D25 +31.3° (in chloroform-methanol, 8:2). Exhibits intense blue fluorescence under uv light. uv max: 220, 254, 290, 370 nm (e 37320, 29230, 4980, 19900). Does not form stable salts with acids. Poorly sol in water.
Melting point: mp 275-277° (Volkmann); 287-288° (Stork, Schultz)
Optical Rotation: [a]D25 +31.3° (in chloroform-methanol, 8:2)
Absorption maximum: uv max: 220, 254, 290, 370 nm (e 37320, 29230, 4980, 19900)
Derivative Type: Acetate
Molecular Formula: C22H18N2O5
Molecular Weight: 390.39
Percent Composition: C 67.68%, H 4.65%, N 7.18%, O 20.49%
Properties: Crystals, dec 271-274°. uv max: 220, 254, 290, 360-370 nm (e 39010, 28740, 6160, 22000).
Absorption maximum: uv max: 220, 254, 290, 360-370 nm (e 39010, 28740, 6160, 22000)
Derivative Type: Chloroacetate
Molecular Formula: C22H17ClN2O5
Molecular Weight: 424.83
Percent Composition: C 62.20%, H 4.03%, Cl 8.35%, N 6.59%, O 18.83%
Properties: Crystals, dec 245-248°.