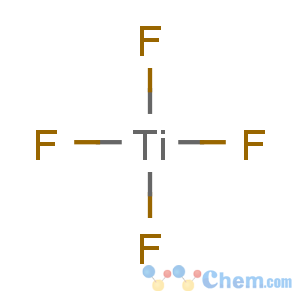
Title: Titanium Tetrafluoride
CAS Registry Number: 7783-63-3
Molecular Formula: F4Ti
Molecular Weight: 123.86
Percent Composition: F 61.35%, Ti 38.65%
Line Formula: TiF4
Literature References: Prepd by the reaction of hydrogen fluoride with titanium tetrachloride: Ruff, Ipsen,
Ber. 36, 1777 (1903); Ruff, Plato,
ibid. 37, 673 (1904); by the action of fluorine on the metal or the dioxide: Haendler
et al., J. Am. Chem. Soc. 76, 2177 (1954).
Properties: Powdery white mass. d420 2.798. Sublimes at 284°. mp >400°. Hisses on contact with water. Very hygroscopic; the dihydrate may be crystallized from aqueous soln (also obtained from solns of TiO2 in HF). Aq solns hydrolyze slowly, and the existence of an oxyfluoride, TiOF2, has been established. Also sol in alcohol and pyridine from which the compds TiF4.2EtOH and TiF4.C5H5N have been isolated. Insol in ether. Dry ammonia is absorbed at room temp to form TiF4.4NH3, but at 120° TiF4.2NH3 is the stable phase. This is sol in water and is sufficiently stable to sublime. TiF4 is also sol in phosphorus oxychloride, but at 30° a reaction occurs and POF3 is evolved.
See also Emeléus in
Fluorine Chemistry vol. I, J. H. Simons, Ed. (Academic Press, New York, 1950) p 47.
Melting point: mp >400°
Density: d420 2.798