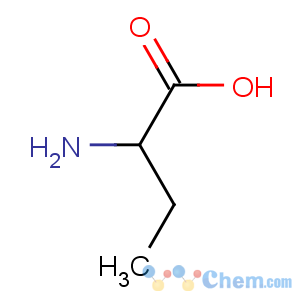
Title: a-Aminobutyric Acid
CAS Registry Number: 80-60-4
CAS Name: 2-Aminobutanoic acid
Synonyms: a-amino-
n-butyric acid
Molecular Formula: C4H9NO2
Molecular Weight: 103.12
Percent Composition: C 46.59%, H 8.80%, N 13.58%, O 31.03%
Line Formula: CH3CH2CH(NH2)COOH
Literature References: Prepn of the DL-form from a-bromobutyric acid and ammonia: Fisher, Mounegrat,
Ber. 33, 2388 (1900); from potassium cyanide, ammonium chloride and propionaldehyde: Zelinsky, Stadnikow,
Ber. 41, 2062 (1908); by reduction of a-oxobutyric acid: Knoop, Oesterlin,
Z. Physiol. Chem. 148, 305 (1925); from a-ethylacetoacetic ester and hydrazoic acid: Schmidt,
Ber. 57, 706 (1924). The L(+)-form has been isolated from proteins: Oikawa,
Chem. Zentralbl. 1926, 1, 148. Configuration: Clough,
J. Chem. Soc. 113, 544, 551; Levene,
Chem. Rev. 2, 203 (1926); Vogler,
Helv. Chim. Acta 30, 1766 (1947).
Derivative Type: DL-Form
Properties: Crystals, mp 304° (begins to sublime when heated above 300°). Sol in water. One liter of water will dissolve 210.5 g at 25°. Sparingly sol in alcohol. One liter of boiling ethanol dissolves about 1.8 g. Insol in ether.
Melting point: mp 304° (begins to sublime when heated above 300°)
Derivative Type: DL-Form ethyl ester
Properties: Viscous liquid. bp11 61°. Sol in water and in organic solvents.
Boiling point: bp11 61°
Derivative Type: L-Form
Properties: Leaflets from dil alc. Sweet taste. mp 270-280° (depending on speed of heating,
see Vogler,
loc. cit.). [M]D +21.2° (5
N HCl); [M]D +43.3° (glacial acetic acid). [a]D16 +8.40° (c = 4); [a]D16 +18.65° (c = 4.8 in 6
N HCl).
Melting point: mp 270-280° (depending on speed of heating,
see Vogler,
loc. cit.)
Optical Rotation: [a]D16 +8.40° (c = 4); [a]D16 +18.65° (c = 4.8 in 6
N HCl)
Derivative Type: L-Form hydrochloride
Properties: Needles; [a]D19 +12.90° (c = 3.64). Readily sol in water.
Optical Rotation: [a]D19 +12.90° (c = 3.64)