Cyanide
-
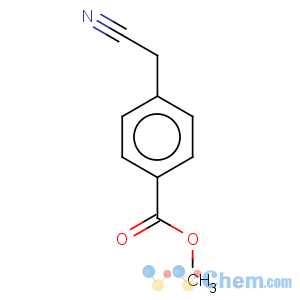
Molecular Structure
Other Information
- CAS Registry Number: 57-12-5
- Transport: 1588
- Flash Point: °C
- Boiling Point: 25.7°Cat760mmHg
- Density: 0.695g/cm3
- Safety Statements: Very poisonous by most routes. Cyanide directly stimulates the chemoreceptors of the carotid and aortic bodies with a resultant hyperpnea (increase in the depth and rate of respiration). Cardiac irregularities are often noted, but the heart invariably outlasts the respirations. Death is due to respiratory arrest of central origin. It can occur within seconds or minutes of the inhalation of high concentrations of HCN gas. Because of slower absorption, death may be more delayed after the ingestion of cyanide salts, but the critical events still occur within the first hour. Two other sources of cyanide have been responsible for human poisoning: the naturally occurring amygdalin and the drug nitroprusside.Amygdalin is a cyanogenic glycoside found in apricot, peach, and similar fruit pits and in sweet almonds (Sayre and Kaymakcalan, 1941). It is a chemical combination of glucose, benzaldehyde, and cyanide from which the latter can be released by the action of β-glucosidase or emulsion. Although these enzymes are not found in mammalian tissues, the human intestinal microflora appears to possess these or similar enzymes capable of effecting cyanide release resulting in human poisoning. For this reason, amygdalin may be as much as 40 times more toxic by the oral route as compared with intravenous injection. Amygdalin is the major ingredient of Laetrile, and this alleged anticancer drug has also been responsible for human cyanide poisoning.An ethical drug that may also cause cyanide poisoning in overdose is the potent vascular smooth-muscle relaxant sodium nitroprusside. Although nitroprusside is related chemically to ferricyanide, unlike the latter it penetrates into erythrocytes and reacts with hemoglobin to release its cyanide (Smith and Kruszyna, 1974). Fortunately, the therapeutic margin for nitroprusside appears to be quite large.Cyanide is commonly found in certain rat and pest poisons, silver and metal polishes, photographic solutions, and fumigating products. Compounds such as potassium cyanide can also be readily purchased from chemical stores. Cyanide is readily absorbed from all routes, including the skin, mucous membranes, and by inhalation, although alkali salts of cyanide are toxic only when ingested. Death may occur with ingestion of even small amounts of sodium or potassium cyanide and can occur within minutes or hours depending on route of exposure. Inhalation of toxic fumes represents a potentially rapidly fatal type of exposure. A blood cyanide level of greater than 0.2 µg/mL is considered toxic. Lethal cases have usually had levels above 1 µg/mL.Clinically, cyanide poisoning is reported to produce a bitter, almond odor on the breath of the patient; however, only a small proportion of the population is genetically able to discern this characteristic odor. Typically, cyanide has a bitter, burning taste, and, following poisoning, symptoms of salivation, nausea without vomiting, anxiety, confusion, vertigo, giddiness, lower-jaw stiffness, convulsions, opisthotonos, paralysis, coma, cardiac arrhythmias, and transient respiratory stimulation followed by respiratory failure may occur. Bradycardia is a common finding, but in most cases heartbeat usually outlasts respiration (Wexler et al., 1947). A prolonged expiratory phase is considered to be characteristic of cyanide poisoning. (Casarett and Doull's, Toxicology, The Basic Science of Poisons 2nd ed. Doull, Klaassen and Amdur (eds), Macmillan Pub. Co. Inc. New York, NY).The volatile cyanides resemble HCN physiologically, inhibiting tissue oxidation and causing death through asphyxia. Cyanogen is probably as toxic as HCN; the nitriles are generally considered somewhat less toxic, probably because of their lower volatility. The non-volatile cyanide salts appear to be relatively nontoxic systemically, so long as they are not ingested and care is taken to prevent the formation of HCN. Workers, such as electroplaters and picklers, who are daily exposed to cyanide solutions may develop a “cyanide” rash, characterized by itching, and by macular, papular, and vesicular eruptions. Frequently there is secondary infection. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes. See also specific cyanide compounds.Flammable by chemical reaction with heat, moisture, acid. Many cyanides rather easily evolve HCN, a flammable gas that is highly toxic. Carbon dioxide from the air is sufficiently acidic to liberate HCN from cyanide solutions. Reaction with hypochlorite solutions may be violent at pH 10.0–10.3. Cyanides explode if melted with nitrites or chlorates at about 450°. Violent reaction with F2, Mg, nitrates, HNO3, nitrites. Metal cyanides are easily oxidized and may be thermally unstable. N-cyano derivatives may be reactive or unstable. Many organic nitriles can be very reactive under the right conditions. When heated to decomposition or on contact with acid, acid fumes, water, or steam, cyanides emit toxic and flammable vapors of CN−. See also HYDROCYANIC ACID.Analytical Methods: For occupational chemical analysis use OSHA: #ID-120 or NIOSH: Cyanides (Aerosol and Gas) 7904.
- Flash Point: °C
- EINECS: 200-821-6
- Molecular Weight: 0
- InChI: InChI=1/CN/c1-2/q-1
- Molecular Formula: CHN

 View Contact Detail
View Contact Detail Molecular Structure
Molecular Structure