Paclitaxel
-
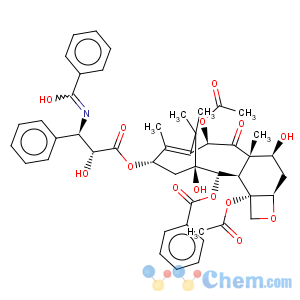
Molecular Structure
Detailed Description
Paclitaxel
Synonyms: N-BENZYL-BETA-PHENYLISOSERINE ESTER;PACLITAXEL, TAXUS BREVIFOLIAPA
CAS: 33069-62-4
MF: C47H51NO14
MW: 853.91
EINECS: 205-285-7
Product Categories: Active Pharmaceutical Ingredients;Pharmaceutical material and intermeidates;Antineoplastics;Antitumors for Research and Experimental Use;Biochemistry;Natural Plant Extract;Intermediates & Fine Chemicals;Pharmaceuticals;Antitumour;Signalling;Aromatics;Chiral Reagents;Heterocycles;chemical reagent;pharmaceutical intermediate;phytochemical
Chemical Properties: White Powder
Assay:99%
Usage: glucocorticoid, antiinflammatory .An antineoplastic. Used in the study of structure and function of microtubles into tubulin. Paclitaxel is now used to treat patients with lung, ovarian, breast cancer, head and neck cancer, and advanc ed forms of Kaposi's sarcoma. Paclitaxel is a mitotic inhibitor used in cancer chemotherapy.
Packing:as required
TEST TEST METHOD SPECIFICATIONS RESULT
ASSAY (calculated on the anhydrous, solvent-free basis) USP<621> 97.0%~102.0% 99.7%
IDENTIFICATION IR USP<197K> Infrared Absorption:In accordance with the reference Standard spectrum of paclitaxel Conforms
HPLC USP<621> The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay. Conforms
SPECIFIC ROTATION USP<781S>Method Ic -49.0??~-55.0° -53.3??
RELATED COMPOUNDS (HPLC) 10-Deacetyl baccatin III USP<621>Test NMT 0.1% Undetected
Baccatin III NMT 0.2% 0.031%
10-Deacetylpaclitaxel NMT 0.5% Undetected
Photodegradant NMT 0.1% Undetected
10,13-Bissidechainpaclitaxel NMT 0.5% Undetected
7-Acetylpaclitaxel NMT 0.6% Undetected
7-Tes-paclitaxel NMT 0.3% Undetected
13-Tes-baccatin III NMT 0.1% Undetected
7-Epipaclitaxel NMT 0.4% 0.049%
2-Debenzoylpaclitaxel-2-pentenoate NMT 0.7% Undetected
10-Deacetyl-7-cpipaclitaxel NMT 0.4% 0.0076%
Others NMT 0.1% (each) 0.010%
Total NMT 2.0% 0.11%
WATER USP<921>Method Ic NMT 4.0% 2.2%
RESIDUE ON IGNITION USP<281> NMT 0.2% Conforms
HEAVY METALS USP<231>Method¢ò NMT 0.002% Conforms
BACTERIAL ENDOTOXINS USP<85> NMT 0.4 EU/mg Conforms
MICROBIAL LIMITS <> USP<61> Total aerobic microbial count NMT100 cfu/g 10 cfu/g
It meets the requirements of the tests for the absence of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella species, and Escherichia coli Undetected
CONCLUSION he product is in accordance withUSP34 to test the above-mentioned items, The test results conform to the specification

- Paclitaxel




