Doxorubicin hydrochloride
-
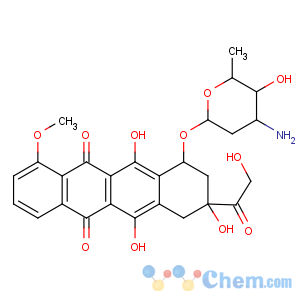
Molecular Structure
Detailed Description
Doxorubicin hydrochloride
Synonyms:Adriacin;Adriblastina;Adriblastina hydrochloride;Adriamycin hcl;Adriamycin hydrochloride;(8s-cis)-10-[(3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxynaphthacene-5,12-dione hydrochloride;14-hydroxydaunomycin hcl;14-hydroxydaunomycin hydrochloride
Cas:25316-40-9
Mf:C27h30clno11
Mw:579.98
Einecs:246-818-3
Product categories:Apis;Active pharmaceutical ingredients;Pharmaceuticals;Antitumour;Adriamycin;Inhibitor;Anti-cancer&immunity
General description:Orange-red thin needles.Aqueous solutions yellow-orange at acid phs, orange-red at neutral phs, and violet blue over ph 9.
Assay:99%
Air & water reactions:Water soluble.
Usage:Doxorubicin hydrochloride (adriamycin hydrochloride) is an antitumor agent that has been formulated as a salt to achieve higher water solubility.While the salt shares the same pharmacological properties as doxorubicin free base, its greater water solubility may offer advantages in some in vitro applications.Physicochemical properties and chromatographic behaviour will depend on whether the ph is buffered.In non-ph controlled systems the free base and salt may behave differently.
Packing:Foil bag or as required
Product Name Doxorubicin hydrochloride(C27H30ClNO11) CAS:25316-40-9
Items Tested Specification Result
Purity 98.0~102.0% 99.02%
Description Orange-red or red crystalline powder; No visible evidence of contamination by foreign matter. Confirm
Identification A IR spectrum corresponds to the IR spectrum of the reference standard Confirm
Identification B The retention time of the doxorubicin peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay. Confirm
Solubility Soluble in water and methanol; slightly soluble in ethanol; practically insoluble in acetone. Conforms
Residual Solvents
Acetone ≤0.5% 0.31%
Acetone +Ethanol ≤2.5% 0.60%
methanol ≤0.3% Not detected
Dichloromethane ≤0.06% Not detected
pH 4.0 to 5.5 5.1
Water Content ≤4.0% 0.8%
Related Substances
Impurity A (Doxorubicinone) ≤0.5% 0.1%
Any Other unknown Impurity ≤ 0.1% 0.07%
Total unknown Impurities ≤ 0.3% 0.2%
Total Impurities ≤1.0% 0.9%
Conclusion It complies to reference standard

- Doxorubicin hydrochloride




