Paclitaxel pure and high quality
-
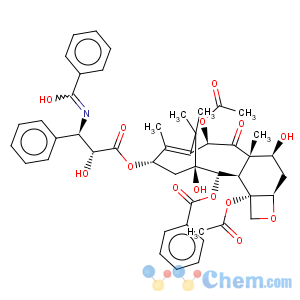
Molecular Structure
Detailed Description
supply: 4cec 4cdc 4mpb bkebdp fubamb 5fabd mmbc
5F-ADB HEXEN, fubamb,4-cec,4-DCD,bk-ebdp,STG78,5fmdmb2201,4-mpd,4CLMPD,MDPHP,4eec,5cabp,mdpt,bmdp,2-fdck,mmaf
buff BK-ebdp fubamb 5fadb Bromadoline 2fdck
Skype:+86 17037496173
E-mail:cindy@hbmeihua.cn
WhatsApp:cindy@hbmeihua.cn
Paclitaxel is approved in the UK for ovarian, breast and lung, bladder, prostate, melanoma, esophageal, and other types of solid tumor cancers as well as Kaposi's sarcoma. It is recommended in NICE guidance of June 2001 that it should be used for nonsmall cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should not be used in the adjuvant treatment of early node-positive breast cancer. In 2005, its use in the United States for the treatment of breast, pancreatic, and non-small cell lung cancers was approved by the FDA.

- Paclitaxel pure and high quality




