Contact us
- Company Name: Guangzhou Tengyue Chemical Co., Ltd.
- Street: 7th Ladder 208, Section 8 of Luojia Village, Fuyi Road, Dalong Street, Panyu District, Guangzhou, Guangdong, China
- City: Guangzhou
- Province/state: Guangdong
- Country/region: China
- Contact Person: Ms.Judy Wang
- Tel: 86-020-18102676775
- Fax: 86-020-28165197
- Email:

Vardenafil
-
- Product NameVardenafil
- CAS No.224785-91-5
- Purity
- Min Quantity
- Price~

 View Contact Detail
View Contact Detail
-
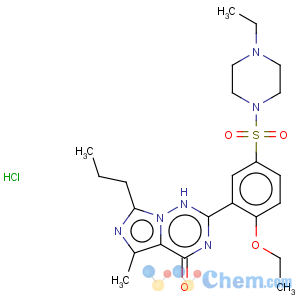 Molecular Structure
Molecular Structure
Detailed Description
VardenafilCAS No. 224785-91-5
Molecular formula: C23H33ClN6O4S
Molecular weight: 525.06
Vardenafil hydrochloride is a first-line treatment for erectile dysfunction in men. It was developed by German Bayer Company and first listed in the United States in 2002. Like sildenafil, it belongs to the second generation of phosphodiesterase type 5 inhibitors. It works very quickly and takes effect in about 15-20 minutes. The effect was ten times stronger than sildenafil. Clinical data show that 50% to 60% of male diabetic patients over 50 years old suffer from erectile dysfunction, especially those caused by nerve and blood vessel injury caused by diabetes mellitus. Vardenafil, a potent selective phosphodiesterase 5 (PDE5) inhibitor developed by Bayer, may bring good news to patients with erectile dysfunction caused by diabetes. These drugs are used as disposable therapeutic drugs. After oral administration about 1 hour before sexual life, the concentration of cGMP induced by nitric oxide (NO) released after sexual stimulation is increased by selective inhibition of PDE5 effect, relaxation of arterial smooth muscle, enhancement of erectile function, occasional headache, blush, stomach discomfort and color. Adverse reactions such as sensory disturbance.
Three selective 5-type phosphodiesterase (PDE5) inhibitors: sildenafil, valdenafil hydrochloride and tadalafil are all first-line drugs for ED. There is no significant difference between PDE5 inhibitors and apomorphine in efficacy, safety and tolerance of drugs. E1 is the second-line treatment for ED, and the effectiveness of intraurethral prostaglandin E1 is worse than that of intracavernous injection of prostaglandin E1. Conclusion PDE5 inhibitors are effective, well tolerated and safe in the treatment of ED. Apomorphine, intracavernous injection of prostaglandin E1 and intraurethral administration of prostaglandin E1 are also effective and well tolerated.