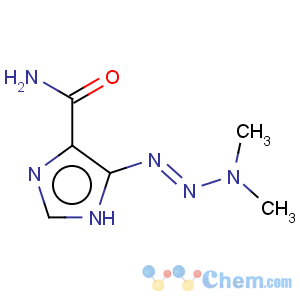
Title: Dacarbazine
CAS Registry Number: 4342-03-4
CAS Name: 5-(3,3-Dimethyl-1-triazenyl)-1
H-imidazole-4-carboxamide
Synonyms: 5(or 4)-(dimethyltriazeno)imidazole-4(or 5)-carboxamide; DIC; DTIC
Manufacturers' Codes: NSC-45388
Trademarks: Dacatic (L×kefarmos); DTIC-Dome (Bayer); Deticene (Bellon)
Molecular Formula: C6H10N6O
Molecular Weight: 182.18
Percent Composition: C 39.56%, H 5.53%, N 46.13%, O 8.78%
Literature References: First synthesized in 1959 at the Southern Research Institute. Prepn: Shealy
et al., J. Org. Chem. 27, 2150 (1962); Hano
et al., Gann 59, 207 (1968),
C.A. 69, 42527g (1968). Antitumor activity: Shealy
et al., Biochem. Pharmacol. 11, 674 (1962); Hano
et al., Gann 56, 417 (1965),
C.A. 63, 18856g (1965). Mechanism of action studies: Saunders, Schultz,
Biochem. Pharmacol. 19, 911 (1970). Metabolism: Skibba
et al., ibid. 2043; Mizuno, Humphrey,
Cancer Chemother. Rep. Part 1 56, 465 (1972).
Review: Carter, Friedman,
Eur. J. Cancer 8, 85-92 (1972),
see also the series of articles on history, activity, mechanism of action and clinical studies:
Cancer Treat. Rep. 60, 123-214 (1976).
Properties: Ivory microcrystalline substance; explosive decomp 250-255°. Also reported as mp 205°, Hano
et al., loc. cit. (1965). uv max (0.1
N HCl): 223 nm (7500); (pH 7): 237 nm (11200); both solns protected from light. Stable in neutral soln in absence of light.
Melting point: mp 205°, Hano
et al., loc. cit. (1965)
Absorption maximum: uv max (0.1
N HCl): 223 nm (7500); (pH 7): 237 nm (11200); both solns protected from light
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-76.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkylating Agents.