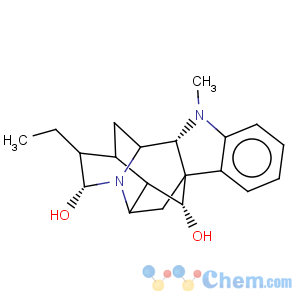
Title: Ajmaline
CAS Registry Number: 4360-12-7
CAS Name: Ajmalan-17,21-diol
Synonyms: rauwolfine
Trademarks: Aritmina (Altana); Gilurytmal (Solvay); Ritmos (Sanofi-Synthelabo); Tachmalin (AWD)
Molecular Formula: C20H26N2O2
Molecular Weight: 326.43
Percent Composition: C 73.59%, H 8.03%, N 8.58%, O 9.80%
Literature References: From roots of
Rauwolfia serpentina (L.) Benth.
(Ophioxylon serpentinum L.),
Apocynaceae. Isolation: S. Siddiqui, R. H. Siddiqui,
J. Indian Chem. Soc. 8, 667 (1931);
9, 539 (1932);
12, 37 (1935); L. van Itallie, A. J. Steenhauer,
Arch. Pharm. 270, 313 (1932). Structure: A. Chatterjee, S. Bose,
J. Indian Chem. Soc. 31, 17 (1954); F. A. L. Anet
et al., J. Chem. Soc. 1954, 1242. Stereochemistry: M. F. Bartlett
et al., J. Am. Chem. Soc. 84, 622 (1962). Synthesis: S. Masamune
et al., ibid. 89, 2506 (1967); E. E. Van Tamelen, L. K. Oliver,
ibid. 92, 2136 (1970); K. Mashimo, Y. Sato,
Chem. Pharm. Bull. 18, 353 (1970). Physico-chemical properties: A. Petter,
Arzneim.-Forsch. 24, 874 (1974). Antiarrhythmic activity: A. Petter, K. Engelmann,
ibid. 876.
Reviews: R. Robinson in
Festschrift Arthur Stoll (Birkh?user-Verlag, Basel, 1957) pp 457-467; A. Koskinen, M. Lounasmaa in
Progress in the Chemistry of Natural Products vol. 43, W. Herz
et al., Eds. (Springer-Verlag, New York, 1983) pp 268-346.
Properties: Pale amber, solvated, tetragonal prisms from methanol, C20H26N2O2.CH3OH, mp 158-160°. [a]D18 +131° (c = 0.4 in chloroform). Anhydr mp 205-207°. [a]D20 +144° (c = 0.8 in chloroform). uv max (ethanol): 247, 295 nm (log e 3.94, 3.49). Sol in methanol, ethanol, ether, chloroform; slightly sol in water.
Melting point: mp 158-160°; mp 205-207°
Optical Rotation: [a]D18 +131° (c = 0.4 in chloroform); [a]D20 +144° (c = 0.8 in chloroform)
Absorption maximum: uv max (ethanol): 247, 295 nm (log e 3.94, 3.49)
Derivative Type: Hydrochloride dihydrate
Molecular Formula: C20H26N2O2.2HCl.2H2O
Molecular Weight: 435.39
Percent Composition: C 55.17%, H 7.41%, N 6.43%, O 14.70%, Cl 16.29%
Properties: Hexagonal bipyramidal crystals from water, mp 140°. [a]D18 +96.6°. One gram dissolves in 40 ml water.
Melting point: mp 140°
Optical Rotation: [a]D18 +96.6°
Therap-Cat: Antihypertensive; antiarrhythmic.
Keywords: Antiarrhythmic; Antihypertensive.