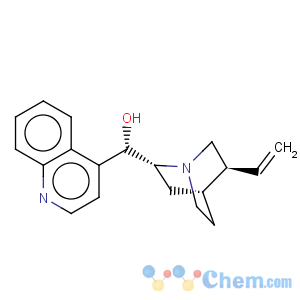
Title: Cinchonidine
CAS Registry Number: 485-71-2
CAS Name: (8a,9
R)-Cinchonan-9-ol
Synonyms: cinchovatine; a-quinidine
Molecular Formula: C19H22N2O
Molecular Weight: 294.39
Percent Composition: C 77.52%, H 7.53%, N 9.52%, O 5.43%
Literature References: Occurs in most varieties of cinchona bark, especially in bark of
Cinchona pubescens Vahl. (
C. succirubra Pav.) and
C. pitayensis Wedd.,
Rubiaceae. Isoln: Leers,
Ann. 82, 147 (1852). Structure: Rabe,
ibid. 365, 359 (1909). Stereoisomeric with cinchonine: Koenigs,
ibid. 347, 182 (1906). Configuration: Prelog, Zalán,
Helv. Chim. Acta 27, 535 (1944); Prelog, H?fliger,
ibid. 33, 2021 (1950); Roth,
Pharmazie 16, 257 (1961); Lyle, Keefer,
Tetrahedron 23, 3253 (1967). Biosynthetic studies: Battersby, Parry,
Chem. Commun. 1971, 31. Toxicity studies: C. C. Johnson, C. F. Poe,
Acta Pharmacol. Toxicol. 4, 265 (1948); E. W. Schafer, Jr.
et al., Ecotoxicol. Environ. Saf. 6, 149 (1982).
Properties: Orthorhombic plates, prisms from alcohol, mp 210°. [a]D20 -109.2° (alc). uv absorption data: Kamath
et al., Indian J. Chem. 6, 510 (1968). Sol in alcohol and chloroform; moderately sol in ether. Practically insol in water. pK1 5.80, pK2 10.03.
Protect from light. LD50 i.p. in rats: 206 mg/kg (Johnson, Poe). LD50 orally in quail: >316 mg/kg (Schafer).
Melting point: mp 210°
pKa: pK1 5.80, pK2 10.03
Optical Rotation: [a]D20 -109.2° (alc)
Toxicity data: LD50 i.p. in rats: 206 mg/kg (Johnson, Poe); LD50 orally in quail: >316 mg/kg (Schafer)
Derivative Type: Dihydrochloride
Molecular Formula: C19H22N2O.2HCl
Molecular Weight: 367.31
Percent Composition: C 62.13%, H 6.59%, N 7.63%, O 4.36%, Cl 19.30%
Properties: White or slightly yellow crystals or powder. Freely sol in water or alcohol.
Protect from light.
Derivative Type: Hydrochloride dihydrate
Molecular Formula: C19H22N2O.HCl.2H2O
Molecular Weight: 366.88
Percent Composition: C 62.20%, H 7.42%, N 7.64%, O 13.08%, Cl 9.66%
Properties: Cryst powder; loses all of its H2O at 120°. [a]D20 -117.5° (water). Sol in 25 parts cold water, more sol in boiling water; sol in alcohol, chloroform, slightly in ether. The aq soln is practically neutral.
Protect from light.
Optical Rotation: [a]D20 -117.5° (water)
Derivative Type: Sulfate trihydrate
Molecular Formula: (C19H22N2O)2.H2SO4.3H2O
Molecular Weight: 740.91
Percent Composition: C 61.60%, H 7.07%, N 7.56%, O 19.43%, S 4.33%
Properties: Silky, acicular crystals; efflorescent on exposure to air and darkens in light. mp when anhydr ~240° with decompn. One gram dissolves in 70 ml water, 20 ml hot water, 90 ml alcohol, 40 ml hot alc, 620 ml chloroform. Practically insol in ether. The aq soln is practically neutral.
Protect from light.
Melting point: mp when anhydr ~240° with decompn
Derivative Type: Epicinchonidine
CAS Registry Number: 550-54-9
CAS Name: (8a,9
S)-Cinchonan-9-ol
Properties: mp 104°, [a]D20 +63° (c = 0.804 in alc): Rabe
et al., Ann. 492, 253 (1932).
Melting point: mp 104°
Optical Rotation: [a]D20 +63° (c = 0.804 in alc): Rabe
et al., Ann. 492, 253 (1932)
Therap-Cat: Antimalarial.
Keywords: Antimalarial.