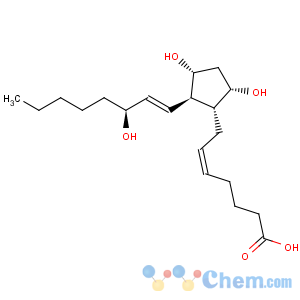
Title: Prostaglandin F2a
CAS Registry Number: 551-11-1
CAS Name: (5
Z,9a,11a,13
E,15
S)-9,11,15-Trihydroxyprosta-5,13-dien-1-oic acid
Synonyms: 7-[3,5-dihydroxy-2-(3-hydroxy-1-octenyl)cyclopentyl]-5-heptenoic acid; dinoprost; PGF2a
Manufacturers' Codes: U-14583
Trademarks: Enzaprost F (Ceva); Prostarmon F (Ono)
Molecular Formula: C20H34O5
Molecular Weight: 354.48
Percent Composition: C 67.77%, H 9.67%, O 22.57%
Literature References: One of the most biologically studied of the primary prostaglandins. Closely related to prostaglandin E2 (PGE2) in that both prostaglandins are biosynthesized from the same precursors and that PGF2a is the synthetic reduction product of PGE2. For refs to synthesis of
dl and natural forms
see Prostaglandin E2. Prepn of the tromethamine salt: W. Morozowich,
DE 2126127;
idem, US 3657327 (1971, 1972 both to Upjohn). Alternate synthesis of natural PGF2a: Schneider, Murray,
J. Org. Chem. 38, 397 (1973); R. B. Woodward
et al., J. Am. Chem. Soc. 95, 6853 (1973); G. Stork
et al., ibid. 100, 8272 (1978); K. Kondo
et al., Tetrahedron Lett. 1978, 3927; N. R. A. Beeley
et al., Tetrahedron 37, Suppl. 9, 411 (1981); R. J. Cave
et al., J. Chem. Soc. Perkin Trans. 1 1981, 646. Causes vasocontraction and exhibits luteolytic activity; is most commonly associated with its role in pregnancy: Karim
et al., J. Obstet. Gynaecol. Brit. Commonw. 78, 172 (1971). Metabolism in female subjects: Granstrom, Samuelsson,
J. Biol. Chem. 246, 5254 (1971). Toxicity data: T. Fujita
et al., Iyakuhin Kenkyu 9, 261 (1978),
C.A. 89, 71399k (1978). For general refs
see Prostaglandins.
Properties: Natural form, crystals, mp 25-35°. [a]D25 +23.5° (c = 1 in tetrahydrofuran). Freely sol in methanol, abs ethanol, ethyl acetate, chloroform; slightly sol in water. Stable for two years in light resistant containers at 5-15°. Degrades in one week when exposed to sunlight or in three months at 40°. LD50 in rabbits (mg/kg): 2.5-5.0 i.v.; 2.5-5.0 i.m. (Fujita).
Melting point: mp 25-35°
Optical Rotation: [a]D25 +23.5° (c = 1 in tetrahydrofuran)
Toxicity data: LD50 in rabbits (mg/kg): 2.5-5.0 i.v.; 2.5-5.0 i.m. (Fujita)
Derivative Type: Tromethamine salt
CAS Registry Number: 38562-01-5
Trademarks: In-Synch (ProLabs); Lutalyse (Pharmacia & Upjohn)
Molecular Formula: C20H34O5.C4H11NO3
Molecular Weight: 475.62
Percent Composition: C 60.61%, H 9.54%, O 26.91%, N 2.94%
Properties: White or off-white cystalline powder, mp 100-101°. Readily sol in water to at least 200 mg/ml.
Melting point: mp 100-101°
Therap-Cat: Oxytocic; abortifacient.
Therap-Cat-Vet: Oxytocic.
Keywords: Abortifacient/Interceptive; Oxytocic; Prostaglandin/Prostaglandin Analog.