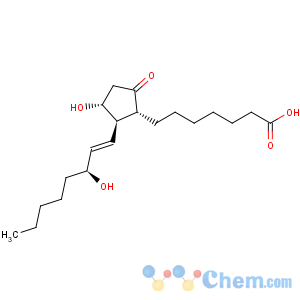
Title: Prostaglandin E1
CAS Registry Number: 745-65-3
CAS Name: (11a,13
E,15
S)-11,15-Dihydroxy-9-oxoprost-13-en-1-oic acid
Synonyms: 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic acid; alprostadil; PGE1
Manufacturers' Codes: U-10136
Trademarks: Caverject (Pharmacia & Upjohn); Edex (Schwarz); Liple (Mitsubishi); Liprostin (Endovasc); Minprog (Pharmacia); Muse (Meda); Palux (Taisho); Prostandin (Ono); Prostin VR (Pharmacia & Upjohn); Prostivas (Pharmacia & Upjohn)
Molecular Formula: C20H34O5
Molecular Weight: 354.48
Percent Composition: C 67.77%, H 9.67%, O 22.57%
Literature References: A primary prostaglandin; easily crystallized from purified biological extracts. Isoln from sheep seminal vesicle tissue, and structure: Bergstrom
et al., Acta Chem. Scand. 16, 501 (1962);
eidem, J. Biol. Chem. 238, 3555 (1963). Enzymic conversion from 8,11,14-eicosatrienoic acid: Nugteren
et al., Rec. Trav. Chim. 85, 405 (1966). Synthesis of the
dl-form: Corey
et al., J. Am. Chem. Soc. 90, 3245, 3247 (1968); Schneider
et al., ibid. 5895;
91, 5372 (1969); Axen
et al., Chem. Commun. 1969, 303; Taub
et al., ibid. 1970, 1258; Slates
et al., ibid. 1972, 304; Kuo
et al., Tetrahedron Lett. 1972, 5317; Taub
et al., Tetrahedron 29, 1447 (1973); Miyano, Stealey,
Chem. Commun. 1973, 180; Finch
et al., J. Org. Chem. 38, 4412 (1973). Synthesis of natural form: Corey
et al., J. Am. Chem. Soc. 91, 535 (1969);
92, 2586 (1970); Sih
et al., ibid. 94, 3643 (1972);
95, 1676 (1973); Schaaf, Corey,
J. Org. Chem. 37, 2921 (1974); Slates
et al., Tetrahedron 30, 819 (1974). Metabolism in guinea pigs: Anggard, Samuelsson,
J. Biol. Chem. 239, 4097 (1964). Metabolism in humans: Hamberg, Samuelsson,
ibid. 246, 6713 (1971). Review of biological activities: Berti
et al., Prog. Biochem. Pharmacol. 3, 110 (1967). Comparative pharmacology with respect to other prostaglandins: Weeks,
Annu. Rev. Pharmacol. 12, 317 (1972). Clinical use in neonates with cyanotic congenital heart disease: P. M. Olley
et al., Adv. Prostaglandin Thromboxane Res. 7, 913 (1980). Use in non-atherosclerotic vasculopathy: D. L. Wooster
et al., J. Am. Med. Assoc. 245, 1846 (1981). Clinical trials in impotence: O. I. Linet, F. G. Ogrinc,
N. Engl. J. Med. 334, 873 (1996); H. Padma-Nathan
et al., ibid. 336, 1 (1997).
Properties: Crystals from ethyl acetate + heptane, mp 115-116°. [a]578 -61.6° (c = 0.56 in tetrahydrofuran). Soly at 35°: 8000 mg/100 ml double distilled water. Easily dehydrated in soln at pHs <4 or >8.
Melting point: mp 115-116°
Optical Rotation: [a]578 -61.6° (c = 0.56 in tetrahydrofuran)
Therap-Cat: Vasodilator (peripheral). In treatment of male erectile dysfunction.
Keywords: Prostaglandin/Prostaglandin Analog; Vasodilator (Peripheral).